Research Establishes Gut Microbiota as Key Factor in Sleep Regulation and as a Novel Target for Treating Sleep Disorders
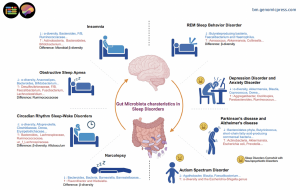
Gut microbiota characteristics in sleep disorders. The figure shows representative characteristics of gut microbiota changes in patients with sleep disorders and those with sleep disorders combined with psychiatric disorders.
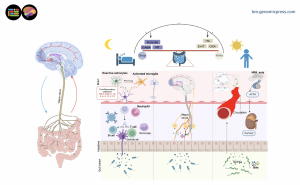
Schematic representation of sleep-microbiome interactions through the microbiota-gut-brain axis. Signals originating from the gut microbiome influence the sleep-wake cycle by modulating the flip-flop switch that governs these states.
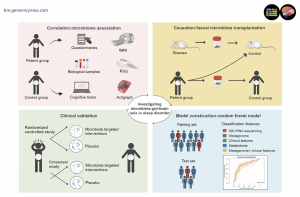
Investigational approaches to the sleep-brain-gut-axis. Methods for investigating brain-gut axis mechanisms in sleep and potential therapeutic approaches. We propose a 4-tiered funnel approach to establish the link between microbiota and sleep disorders.
International Study Reveals Promising Results from Probiotics, Prebiotics, and Microbiome-Based Interventions for Millions Affected by Sleep Disorders
Sleep disorders affect millions worldwide, with conditions ranging from chronic insomnia and obstructive sleep apnea to circadian rhythm disturbances significantly impacting physical health, cognitive function, and emotional well-being. Despite sleep being recognized as a fundamental physiological cornerstone of life, the full complexity of sleep regulation remains incompletely understood. While substantial advances have illuminated central nervous system mechanisms regulating sleep, this review reveals the crucial yet often overlooked role of peripheral organs, particularly the digestive system—in modulating brain function and behavior.
How does the microbiota-gut-brain connection influence sleep? The human gut hosts a diverse ecosystem of microorganisms that communicate bidirectionally with the central nervous system through multiple pathways. These include direct neuronal connections via the vagus nerve, immune system signaling, and the production of bioactive metabolites that can cross the blood-brain barrier. "The gut microbiota is increasingly recognized as a key player in neurological and psychiatric health," explains Professor Lu. "Our review demonstrates that disruptions in gut microbiota composition are closely linked to sleep disturbances across multiple disorders."
The research team examined evidence from both human clinical studies and animal models, revealing consistent patterns of microbial dysbiosis, an imbalance in gut bacterial communities, in individuals with sleep disorders. Notably, patients with chronic insomnia show decreased microbial diversity and altered abundances of specific bacterial families compared to healthy controls. Similar patterns emerge in obstructive sleep apnea, where reduced levels of beneficial bacteria correlate with disease severity. Recent advances in microbiome research have moved beyond simple correlational studies to hypothesis-driven investigations uncovering molecular-level connections between microbiome and sleep-related conditions.
What mechanisms link gut and sleep? The review identifies several biological pathways through which gut microbiota influences sleep regulation, creating a complex web of metabolic, neurological, and immunological interactions. Microbial metabolites play a central role, with short-chain fatty acids like butyrate demonstrating protective effects against sleep disruption in multiple studies. These compounds, produced through bacterial fermentation of dietary fibers, can modulate inflammation, strengthen intestinal barriers, and influence neurotransmitter systems critical for sleep. Clinical trials have shown that sodium butyrate supplementation enhances sleep quality in patients with active ulcerative colitis, while animal studies demonstrate that butyrate alleviates inflammatory responses and memory impairment induced by sleep deprivation.
Bile acids represent another important microbial metabolite class affecting sleep. The research reveals that chronic insomnia associates with elevated levels of primary bile acids including murocholic acid and norcholic acid, alongside reduced secondary bile acids such as isolithocholic acid, lithocholic acid, and ursodeoxycholic acid. This pattern correlates with specific gut bacteria populations, particularly decreased abundances of Ruminococcaceae species, and may contribute to cardiometabolic disease risk in sleep-deprived individuals.
The microbiota also influences production of neurotransmitters directly involved in sleep regulation. Certain gut bacteria, including strains from Lactobacillus and Bifidobacterium, possess genes encoding glutamate decarboxylase, which facilitates production of gamma-aminobutyric acid (GABA), a primary inhibitory neurotransmitter promoting sleep. Additionally, over ninety percent of the body's serotonin is synthesized in the gut, with gut bacteria serving as major producers. Serotonin concentrations fluctuate rhythmically during the sleep-wake cycle, peaking during wakefulness and reaching their lowest levels during REM sleep. Sleep-deprived mice show altered tryptophan metabolism, the precursor to both serotonin and melatonin—changes that are microbiome-dependent and localized to the gut. The gastrointestinal tract is also the most significant extrapineal source of melatonin, with concentrations reaching up to four hundred times those found in plasma.
What evidence exists across different sleep disorders? The review systematically examines microbial alterations across major sleep disorders, revealing both disorder-specific changes and convergent patterns. In insomnia, the most prevalent sleep disorder, studies involving thousands of participants reveal consistent decreases in beneficial bacterial genera alongside shifts in metabolite profiles. A landmark study of 6,398 participants found significant differences in microbial beta-diversity between chronic insomnia patients and healthy individuals, with chronic insomnia associated with lower levels of specific Ruminococcaceae species. These bacterial changes mediated the inverse association between chronic insomnia and cardiometabolic diseases through bile acid alterations.
Obstructive sleep apnea patients demonstrate reduced alpha-diversity, a measure of microbial ecosystem health, with specific bacterial taxa correlating with clinical severity markers including the apnea-hypopnea index and oxygen saturation parameters. Children and adults with OSA show decreased abundances of Ruminococcaceae, suggesting this may be a relatively robust feature of the condition. Animal models further demonstrate that chronic intermittent hypoxia, mimicking OSA pathophysiology, significantly alters gut microbiota composition while increasing systemic inflammatory markers.
Circadian rhythm disorders, including those experienced by shift workers and individuals with chronic jet lag, show distinct microbial signatures. Human studies of night-shift workers reveal increased abundances of Actinobacteria and Firmicutes at the phylum level, with specific species linked to heightened intestinal permeability and inflammatory indicators exhibiting increases after merely two weeks of night-shift employment. Animal models reveal that circadian misalignment triggers rhythmic oscillations in specific bacterial phyla, suggesting the microbiome adapts to, and potentially perpetuates, disrupted circadian rhythms.
Perhaps most intriguing are findings in narcolepsy and REM sleep behavior disorder. These neurological conditions show significant microbial community differences compared to healthy individuals, with some bacterial abundances correlating with symptom severity and sleep architecture measures. In narcolepsy type 1, patients show increased abundance of Klebsiella and decreased beneficial genera such as Blautia, Barnesiella, and Lactococcus. Given that REM sleep behavior disorder often precedes neurodegenerative diseases like Parkinson's disease by years or decades, these microbial biomarkers may offer early detection opportunities.
How do sleep disorders intersect with neuropsychiatric comorbidity? The review highlights that sleep disturbances commonly accompany neuropsychiatric conditions including depression, anxiety disorders, autism spectrum disorder, and neurodegenerative diseases. In these cases, gut microbiota alterations may contribute to both the primary psychiatric condition and comorbid sleep problems through shared inflammatory and neurotransmitter pathways. For example, specific bacterial genera including Blautia, Coprococcus, and Dorea correlate with sleep quality metrics in patients with major depressive disorder.
Children with autism and sleep disturbances show distinct microbial profiles and metabolite abnormalities, including increased diversity indices alongside decreased abundances of Faecalibacterium and Agathobacter. These children also demonstrated decreased melatonin levels and increased serotonin levels, suggesting neurotransmitter alterations linking gut health to sleep disturbances. In Parkinson's disease, which frequently presents with sleep disorders including REM behavior disorder and insomnia, patients show characteristic gut microbiota alterations. Body-first Parkinson's disease patients, who typically present with nonmotor symptoms including sleep disturbances before motor symptoms, show particularly distinct gut microbiome profiles characterized by increased Escherichia coli and Akkermansia muciniphila alongside decreased short-chain fatty acid-producing commensal bacteria.
What are the therapeutic implications of this research? Building on mechanistic understanding, the research examines emerging microbiota-targeted interventions for improving sleep, ranging from probiotics and prebiotics to fecal microbiota transplantation. Probiotics, live beneficial bacteria, show promise in multiple clinical trials across diverse populations. Specific strains have demonstrated efficacy in improving sleep quality, reducing cortisol levels, and enhancing sleep architecture in patients with chronic insomnia. For instance, Lactobacillus plantarum PS128 improved sleep quality in chronic insomnia patients by enhancing delta power during N3 sleep, reflecting deeper and more restorative sleep. Bifidobacterium breve CCFM1025 significantly reduced cortisol levels and improved subjective sleep quality in individuals with insomnia.
Probiotics have also benefited sleep disturbances in Parkinson's disease patients, with Bifidobacterium animalis subsp. lactis Probio-M8 demonstrating significant improvements in Parkinson's disease sleep scale scores. Additionally, individuals with substance use disorders showed promising results, with Lactobacillus acidophilus producing greater reductions in Pittsburgh Sleep Quality Index scores compared to placebo. Animal studies provide complementary evidence and mechanistic insights, with various probiotic strains enhancing sleep efficiency and diminishing anxious behavior through modulation of neurotransmitter and inflammatory factor levels.
Prebiotics, substrates that selectively nourish beneficial gut bacteria, represent another therapeutic avenue with growing evidence. Studies show prebiotic supplementation can modulate bile acid metabolism, reduce inflammation, and improve sleep metrics following circadian disruption. In randomized controlled trials, partially hydrolyzed guar gum supplementation over twelve weeks significantly improved sleep inventory scores in healthy elderly individuals, while resistant dextrin administered to females with type 2 diabetes led to favorable improvements in sleep quality scores. In animal models, prebiotic diets facilitate faster realignment of NREM sleep during circadian challenges and promote REM sleep recovery after stress.
Synbiotics, combinations of probiotics and prebiotics, may offer synergistic benefits by providing both beneficial microorganisms and their preferred substrates. Recent clinical trials demonstrate that synbiotic formulations significantly improve sleep quality in patients with post-acute COVID-19 syndrome and other conditions characterized by sleep disturbances. One study combining Bifidobacterium and Lactobacillus species with prebiotic inulin and oligosaccharides, plus postbiotic extracts, significantly reduced Pittsburgh Sleep Quality Index scores after eight weeks in participants with sleep disturbances.
Perhaps most dramatically, fecal microbiota transplantation from healthy donors has shown remarkable efficacy in small clinical studies, representing a more comprehensive approach to restoring gut microbiome balance. Patients with chronic insomnia comorbid with other chronic diseases experienced significant improvements in insomnia severity and sleep quality scores following FMT treatment, with increases in the relative abundance of Lactobacillus and Bifidobacterium that exhibited negative correlations with symptom scores. In post-acute COVID-19 syndrome patients with insomnia, FMT resulted in significantly higher insomnia remission rates compared to control groups. Even in pediatric populations, FMT led to a ten percent reduction in sleep disturbance scores in children with autism spectrum disorder.
How do different microbiota-targeted therapies compare, and what should future research prioritize? While no direct head-to-head randomized trials have yet compared different microbiota-targeted therapies, the existing evidence suggests each approach offers distinct advantages. Probiotics demonstrate favorable safety profiles, accessibility, and regulatory acceptance, making them most suitable for widespread clinical use in the near term. Prebiotics similarly offer excellent safety and ease of implementation. Synbiotics combine these benefits while potentially offering enhanced efficacy. Fecal microbiota transplantation, while showing dramatic effects in some patients, faces significant obstacles including donor screening requirements, processing standardization, and regulatory limitations, making it more appropriate for research settings or refractory cases.
The authors propose a systematic framework for advancing microbiome-sleep research through four progressive tiers designed to move from observation to clinical application. The first tier involves establishing associations through multimodal assessments including neuroimaging techniques such as functional magnetic resonance imaging and electroencephalography, combined with sleep evaluations using polysomnography and actigraphy, alongside comprehensive microbiome profiling and metabolomic analyses. The second tier focuses on identifying biomarkers using machine learning integration of multi-omics data to analyze large-scale datasets. The third tier emphasizes establishing causality through fecal microbiota transplantation studies in animal models and human intervention trials. The fourth tier involves developing microbiome-based interventions through rigorous randomized controlled trials to assess therapeutic efficacy in ameliorating sleep disorders.
"While significant progress has been made, important challenges remain," notes Professor Lu. "We need larger, well-controlled clinical trials with standardized methodologies to validate therapeutic approaches and understand individual response variability. Harmonizing techniques across studies will enable meaningful cross-study comparisons and accelerate translation to clinical practice."
In conclusion, this comprehensive review establishes the microbiota-gut-brain axis as a critical yet underappreciated factor in sleep regulation, synthesizing evidence across multiple sleep disorders and neuropsychiatric conditions. The convergent evidence from correlational studies, mechanistic investigations, and therapeutic interventions indicates that gut microbiota dysbiosis both results from and contributes to sleep disturbances, creating potential vicious cycles that perpetuate poor sleep and associated health problems. The identification of convergent alterations across multiple sleep disorders, including increased Firmicutes/Bacteroidetes ratios, elevated Actinobacteria and Collinsella levels, alongside decreased abundances of beneficial genera like Bacteroides, Bifidobacterium, and Faecalibacterium, suggests these changes may represent common microbial underpinnings of disturbed sleep, potentially contributing to systemic inflammation and metabolic dysregulation.
As research continues illuminating these complex interactions, microbiota-targeted interventions represent a promising frontier for addressing the global burden of sleep disorders while potentially offering benefits for overall brain health, metabolic function, and quality of life. A deeper understanding of the relationships between gut microbiota and sleep will pave the way for innovative approaches to managing sleep disorders and enhancing overall brain health, potentially transforming how clinicians approach these prevalent and debilitating conditions.
Article and journal information: The Review Article in Brain Medicine titled "Brain-gut-microbiota interactions in sleep disorders," is freely available via Open Access on 4 November 2025 in Brain Medicine at the following hyperlink: https://doi.org/10.61373/bm025i.0128. The research was supported by STI2030-Major Projects and the National Natural Science Foundation of China.
About Brain Medicine: Brain Medicine (ISSN: 2997-2639, online and 2997-2647, print) is a high-quality medical research journal published by Genomic Press, New York. Brain Medicine is a new home for the cross-disciplinary pathway from innovation in fundamental neuroscience to translational initiatives in brain medicine. The journal's scope includes the underlying science, causes, outcomes, treatments, and societal impact of brain disorders, across all clinical disciplines and their interface.
Visit the Genomic Press Virtual Library: https://issues.genomicpress.com/bookcase/gtvov/
Genomic Press online: https://genomicpress.com/
Ma-Li Wong
Genomic Press
mali.wong@genomicpress.com
Visit us on social media:
LinkedIn
Bluesky
Instagram
Facebook
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.


